Support research. Find a cure. Help children and families fight rare diseases.
Help support research to expedite the development of treatments for rare and ultra-rare childhood neuromuscular diseases.
september 7, 2025:
Welcoming Two New Families to Our Mission
United for our Children
The Bailey & Colella Families Join Our Fight Against LGMD2C
The Dion Foundation is honored to announce that two incredible families have joined us in our fight against Limb-Girdle Muscular Dystrophy 2C (LGMD2C): the Bailey Family and the Colella Family.
Like our own, these families know firsthand the urgency of raising awareness, accelerating research, and supporting children who are living with this devastating disease. Together, we are stronger—and together, we are determined to change the future for kids with LGMD2C.
We are grateful to the Baileys and the Colellas for courageously sharing their journeys, lending their voices, and joining forces with us to fundraise, advocate, and shine a light on the children who inspire this mission.
Meet the families and read their stories:
june 2, 2024:
Atamyo Therapeutics Announces First Patients Dosed with ATA-200 Gene Therapy in LGMD-R5 Clinical Trial
First two patients dosed with ATA-200 gene replacement therapy for LGMD-R5
On-going Phase 1b/2 study evaluating safety, pharmacodynamic and efficacy of ATA-200 in pediatric patients
US trial conducted at the Powell Gene Therapy Center, University of Florida, by Dr. Barry Byrne
Evry, France (June 2, 2025) – Atamyo Therapeutics a clinical-stage biotechnology company focused on the development of new generation gene therapies targeting muscular dystrophies and cardiomyopathies, today announced the dosing with ATA-200 gene therapy of first two patients in a phase 1b/2 clinical study in γ-sarcoglycan related limb-girdle muscular dystrophy Type 2C/R5 (LGMD2C/R5). LGMD2C/R5 affects children and leads to loss of ambulation before adulthood.
The multicenter Phase1b/2 open-label dose escalation study (NCT05973630) evaluates safety, pharmacodynamics, efficacy, and immunogenicity in children receiving intravenous ATA-200, a single-dose Adeno-Associated Virus (AAV) gene therapy carrying the human γ-sarcoglycan transgene. The deployment in the US of Atamyo’s clinical trial of ATA-200 is funded by The Dion Foundation for Children with Rare Diseases. This study has also received regulatory clearance in France and Italy.
“This is an exciting milestone for Atamyo. If this clinical trial is successful, it could have a life-changing impact for patients affected by LGMD-R5” said Stephane Degove, Chief Executive Officer and Co-Founder of Atamyo Therapeutics.
“We are delighted to share in the success of the Dion Foundation and Atamyo’s study of LGMD2C/R5 to complete dosing of the first two study participants” said Dr. Barry Byrne, Associate Chair of Pediatrics and Director of the Powell Gene Therapy Center, University of Florida, in Gainesville, Florida, where the first two patients were dosed, and principal investigator of this trial. “We look forward to assessing the potential benefit of ATA-200 in the first children to receive the product.”
“LGMD-R5 is the most severe form of LGMDs with onset of symptoms during childhood and a phenotype close to Duchenne Muscular Dystrophy. We are thrilled to propose a potentially disease-modifying treatment to these young patients” said Dr Sophie Olivier, Chief Medical Officer of Atamyo.
Read full press release here…
September 7, 2024:
The Dion Foundation is funding the first-ever clinical trial for LGMD 2C
The Dion Foundation and Atamyo Therapeutics Announce a Partnership to Expand into the US Atamyo’s Clinical Trial of ATA-200 Gene Therapy to Treat Limb-Girdle Muscular Dystrophy Type 2C/R5
“We are thrilled by this key partnership and grateful to the Dion Foundation for their financial support which aims to include US patients in the first-in-human trial for ATA-200,” said Stéphane Degove, CEO of Atamyo. “We are already engaged in the preparation of an IND filing in the US for ATA-200.”
Atamyo is a spin-off of Genethon, a non-profit research organization that is a pioneer in developing gene therapies for rare diseases. ATA-200 is based on the research of Atamyo Chief Scientific Officer Isabelle Richard, Ph.D., Research Director at National Center of Scientific Research (CNRS) in France and head of the Progressive Muscular Dystrophies Laboratory at Genethon.
Genethon CEO Frederic Revah observed, “This partnership between the Dion Foundation and Atamyo, a spin-off from Genethon, represents another major milestone in our efforts over the past 30 years to develop gene therapies for rare diseases and bring them to patients worldwide.”
Read full press release here…
From the moment we learned of Peter and Maggie’s diagnoses of LGMD 2C/R5 we have been working tirelessly towards this day. “We are so grateful for the opportunity to establish a partnership with Atamyo to help facilitate bringing their groundbreaking research to a clinical site in the US for the very first clinical trial for children with LGMD2C/R5. This is a monumental step for the entire LGMD community,” said Courtney Dion, Co-Founder and President of the Dion Foundation.
We couldn’t be here today without you!
Your support has given us the opportunity to finance the US expansion of Atamyo’s first-ever clinical trial for LGMD 2C/R5! Now, more than ever, we need your continued support: donate, share, spread the news… every small step counts towards this momentous leap for LGMD!
How the dion foundation started…
PETER & MAGGIE WERE BOTH BORN WITH 10 PERFECT FINGERS AND 10 PERFECT TOES. THEY WERE BOTH DISCHARGED HOME FROM THE HOSPITAL WITH A CLEAN BILL OF HEALTH AND HIT ALL THEIR DEVELOPMENT MILESTONES…
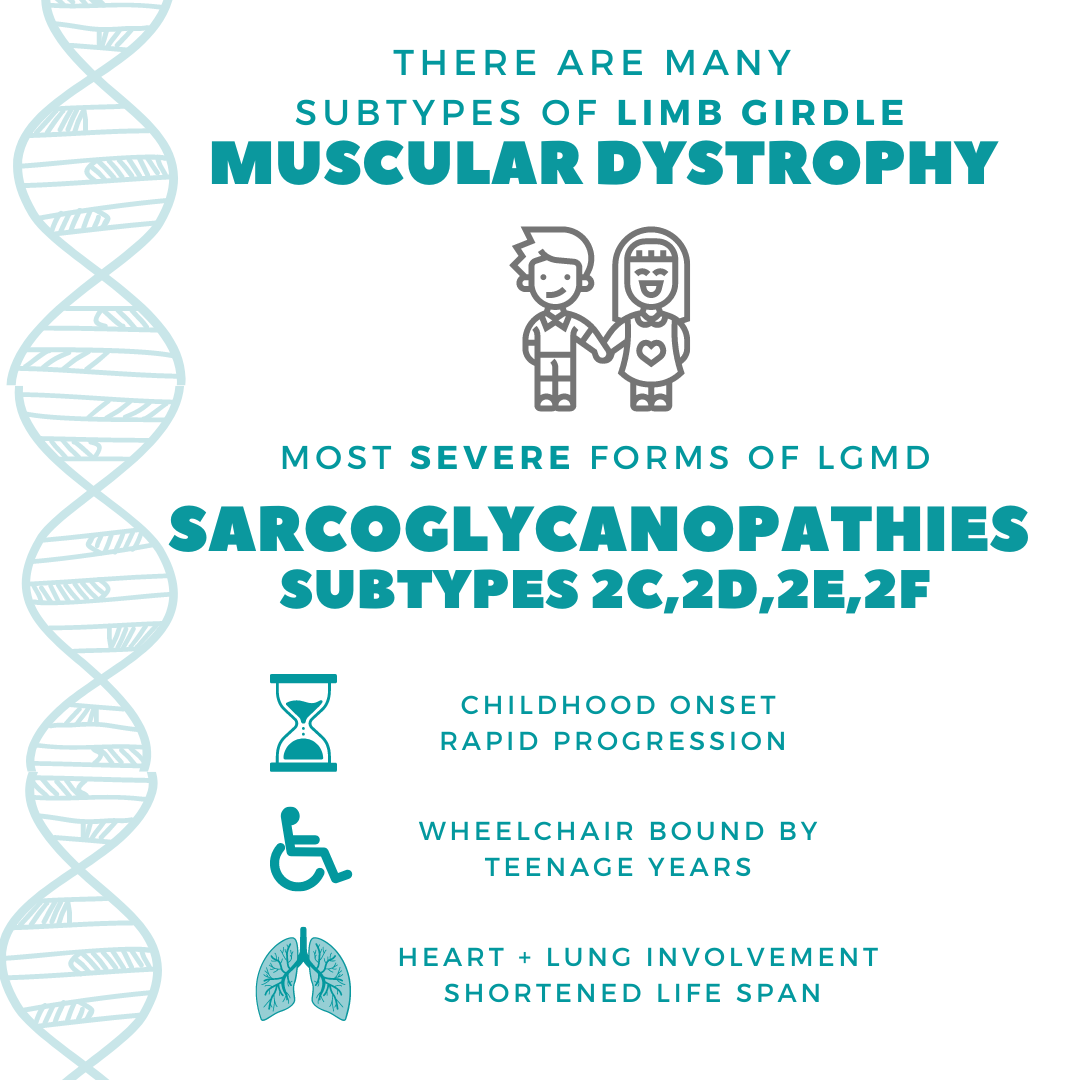
NINE YEARS LATER, EVERYTHING CHANGED WHEN PETER & MAGGIE WERE BE DIAGNOSED WITH A RARE FORM OF MUSCULAR DYSTROPHY LIMB-GIRDLE MUSCULAR DYSTROPHY TYPE 2C (SARCOGLYCANOPATHY)
A RAPIDLY PROGRESSIVE MUSCLE WASTING DISEASE, CAUSED BY GENETIC MUTATIONS. WITH NO CURE.
THUS, THE DION FOUNDATION WAS ESTABLISHED. TO RAISE AWARENESS, FUND RESEARCH AND FIND A TREATMENT FOR THIS FATAL DISEASE.
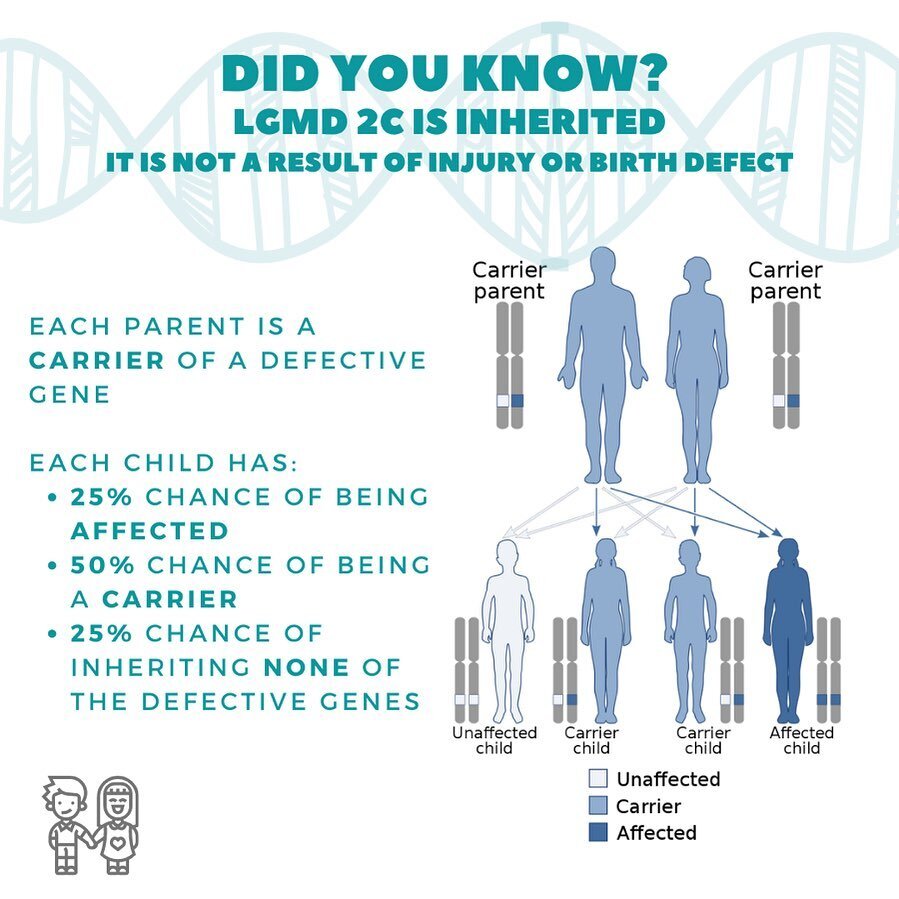
rare without a cure.
what is sarcoglycanopathy?
LIMB GIRDLE MUSCULAR DYSTROPHY TYPES 2C, 2D, 2E, 2F
AGGRESSIVE FORM OF CHILDHOOD MUSCULAR DYSTROPHY
SUPPORT US
WE CAN’T MAKE AN IMPACT WITHOUT YOUR SUPPORT.
TIME & ENERGY
VOLUNTEER TO HELP RAISE AWARENESS IN YOUR COMMUNITY
FOLLOW US